
Neuron-Gale
The galectin-3 Inhibitors project tests a small molecule under development for peripheral indications for treating neuroinflammation in an Alzheimer’s mouse model. By blocking galectin-3, it aims to reduce brain inflammation, with potential in other neurodegenerative diseases. Galectin-3 is involved in neuroinflammation, thereby the inhibitor has a potential to be used in multiple neurodegenerative diseases.
Community Sentiment
How do you feel about this ipt?

Community Sentiment
How do you feel about this ipt?
Loading chart data...
Loading trading interface...
Project
Market Overview
Additional Documentation
Summary
A glycan-binding protein, galectin-3, has been identified as a key regulator of neuroinflammation and postulated as a target for treating Alzheimer’s Disease (AD), supported by the observations that AD patients have significantly increased galectin-3 levels and that galectin-3 knock-out mice have less amyloid plaque deposition and better cognitive functions. In this project a highly potent and specific galectin-3, non brain penetrable inhibitor will be directly infused in the brains in an in vivo AD mouse model to monitor effects of galectin-3 inhibition and potentially validate galectin-3 as a target for pharmacological intervention.
Problem
AD (and Parkinson’s) represents a large unmet medical and societal need with an estimated value of over $30 billion by 2033 (Yahoo finance). Today ~55 million people are affected, and the number is expected to increase. The economic burden is around $1.3 trillion. Current treatments, antibodies or small molecules, are not effective and at best disease-slowing and with side effects. Currently there are no potent galectin-3 inhibitors that pass the blood brain barrier.
Solution
The project aims to test a Galectin-3 inhibitor in a Proof-of-Concept study to mitigate neuroinflammation, Aß plaque deposition in a mouse model of Alzheimer’s disease. An orally available systemic galectin-3 inhibitor for peripheral indications will be infused directly into the brain for a period of four weeks to test the research hypothesis.
Current Status
- Glycomimetic galectin-3 weak-binding inhibitors with high MDCK-MDR1 Papp A-B >40×10-6 cm/sec, which suggests that CNS-available inhibitors can be discovered.
- A soluble glycomimetic galectin-3 high affinity and selective tool inhibitor selected for in vivo Proof of Concept (PoC) in a mouse AD model.
- The tool inhibitor will be chosen based on high affinity for mouse galectin-3, solubility allowing for formulation to high concentrations, which is ideal for Alzet pumps and for intraventricular injections.
- Provided that the proposed mouse in vivo PoC with the tool compound robustly confirms galectin-3 as a drug target in AD, a medicinal chemistry development would be the next step.
Commercial potential
The AD therapeutic market is valued in the tens of billions of dollars annually. A disease-modifying galectin-3 inhibitor with compelling efficacy and safety profile could attain blockbuster drug status, especially if positioned for early-stage or pre-symptomatic AD populations.
Given that the proposed in vivo PoC here confirms galectin-3 as a target and that a subsequent DDD campaign identifies optimal CNS-available galectin-3 inhibitor, the IP will be filed.
Galectin-3 is a novel target in AD. First-mover advantage could be significant if clinical trials show meaningful cognitive or biomarker benefit, particularly with oral, small molecule candidates. This would allow capturing large market share, licensing, partnering, or acquisition interest from major pharma companies.
Market Overview
Additional Documentation
Summary
A glycan-binding protein, galectin-3, has been identified as a key regulator of neuroinflammation and postulated as a target for treating Alzheimer’s Disease (AD), supported by the observations that AD patients have significantly increased galectin-3 levels and that galectin-3 knock-out mice have less amyloid plaque deposition and better cognitive functions. In this project a highly potent and specific galectin-3, non brain penetrable inhibitor will be directly infused in the brains in an in vivo AD mouse model to monitor effects of galectin-3 inhibition and potentially validate galectin-3 as a target for pharmacological intervention.
Problem
AD (and Parkinson’s) represents a large unmet medical and societal need with an estimated value of over $30 billion by 2033 (Yahoo finance). Today ~55 million people are affected, and the number is expected to increase. The economic burden is around $1.3 trillion. Current treatments, antibodies or small molecules, are not effective and at best disease-slowing and with side effects. Currently there are no potent galectin-3 inhibitors that pass the blood brain barrier.
Solution
The project aims to test a Galectin-3 inhibitor in a Proof-of-Concept study to mitigate neuroinflammation, Aß plaque deposition in a mouse model of Alzheimer’s disease. An orally available systemic galectin-3 inhibitor for peripheral indications will be infused directly into the brain for a period of four weeks to test the research hypothesis.
Current Status
- Glycomimetic galectin-3 weak-binding inhibitors with high MDCK-MDR1 Papp A-B >40×10-6 cm/sec, which suggests that CNS-available inhibitors can be discovered.
- A soluble glycomimetic galectin-3 high affinity and selective tool inhibitor selected for in vivo Proof of Concept (PoC) in a mouse AD model.
- The tool inhibitor will be chosen based on high affinity for mouse galectin-3, solubility allowing for formulation to high concentrations, which is ideal for Alzet pumps and for intraventricular injections.
- Provided that the proposed mouse in vivo PoC with the tool compound robustly confirms galectin-3 as a drug target in AD, a medicinal chemistry development would be the next step.
Commercial potential
The AD therapeutic market is valued in the tens of billions of dollars annually. A disease-modifying galectin-3 inhibitor with compelling efficacy and safety profile could attain blockbuster drug status, especially if positioned for early-stage or pre-symptomatic AD populations.
Given that the proposed in vivo PoC here confirms galectin-3 as a target and that a subsequent DDD campaign identifies optimal CNS-available galectin-3 inhibitor, the IP will be filed.
Galectin-3 is a novel target in AD. First-mover advantage could be significant if clinical trials show meaningful cognitive or biomarker benefit, particularly with oral, small molecule candidates. This would allow capturing large market share, licensing, partnering, or acquisition interest from major pharma companies.
Dr. Maryna Polyakova
Maryna uses neuroimaging, neuropsychological testing, and machine learning to improve early diagnostics of PPA. She works at the Foresight Institute, leading the Neurotech Tree project as they build a new roadmap for the future of neurotechnology. She is also a lecturer at HTW Berlin and the University of Leipzig and Max-Planck Research School, where she teaches an introduction to medicine, with a focus on digital health.
Peter Groenen
Industry executive with deep expertise in pharmaceutical R&D, specializing in translational science and biomarker integration in drug discovery and development. Led cross-functional teams advancing biomarker strategies and bridging preclinical and clinical research. Experienced in driving projects from early research to late-stage development, collaborating with academia, industry, and regulatory bodies to accelerate therapeutic innovation. Skilled in data-driven decision-making, alliance management, and strategic problem-solving to navigate scientific and operational challenges.
In vivo POC
In vivo POC - Mouse AD model with ascending dose Alzet pump administered readily available Gal3 inhibitor
Analysing brain by ELISA/WB
Analysing brain by ELISA/WB - Inflammation markers, Aß42 levels.
Analysing brain by immunohistochemistry
Plaque load, Microglia analysis (Iba1, Gal3).
Study data analysis
combining the data from the brain analysis to find a clear inhibitory effect on all the descriptors for AD
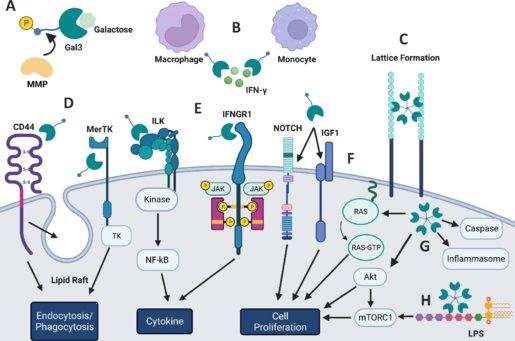
Emerging role of galectin 3 in neuroinflammation and neurodegeneration
https://journals.lww.com/nrronline/fulltext/2024/09000/emerging_role_of_galectin_3_in_neuroinflammation.32.aspx
The role of galectins in immunity and infection. Nat Rev Immunol, 2023
https://www.nature.com/articles/s41577-022-00829-7
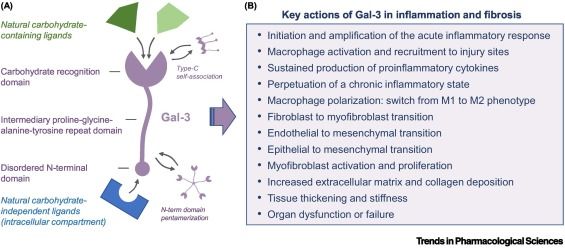
Targeting galectin-3 in inflammatory and fibrotic diseases
https://doi.org/10.1016/j.tips.2023.06.001
Discovery and Optimization of the First Highly Effective and Orally Available Galectin-3 Inhibitors for Treatment of Fibrotic Disease
https://pubs.acs.org/doi/10.1021/acs.jmedchem.2c00660
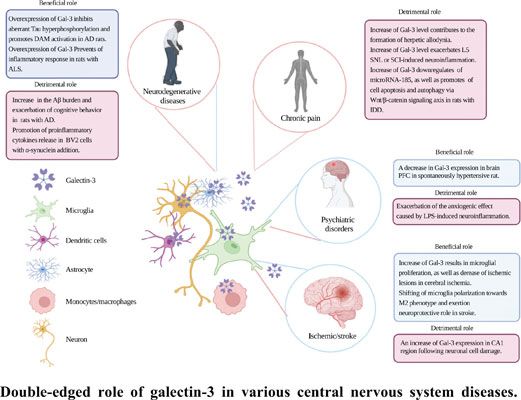
Galectin-3 in Microglia-Mediated Neuroinflammation: Implications for Central Nervous System Diseases
https://www.eurekaselect.com/article/120595
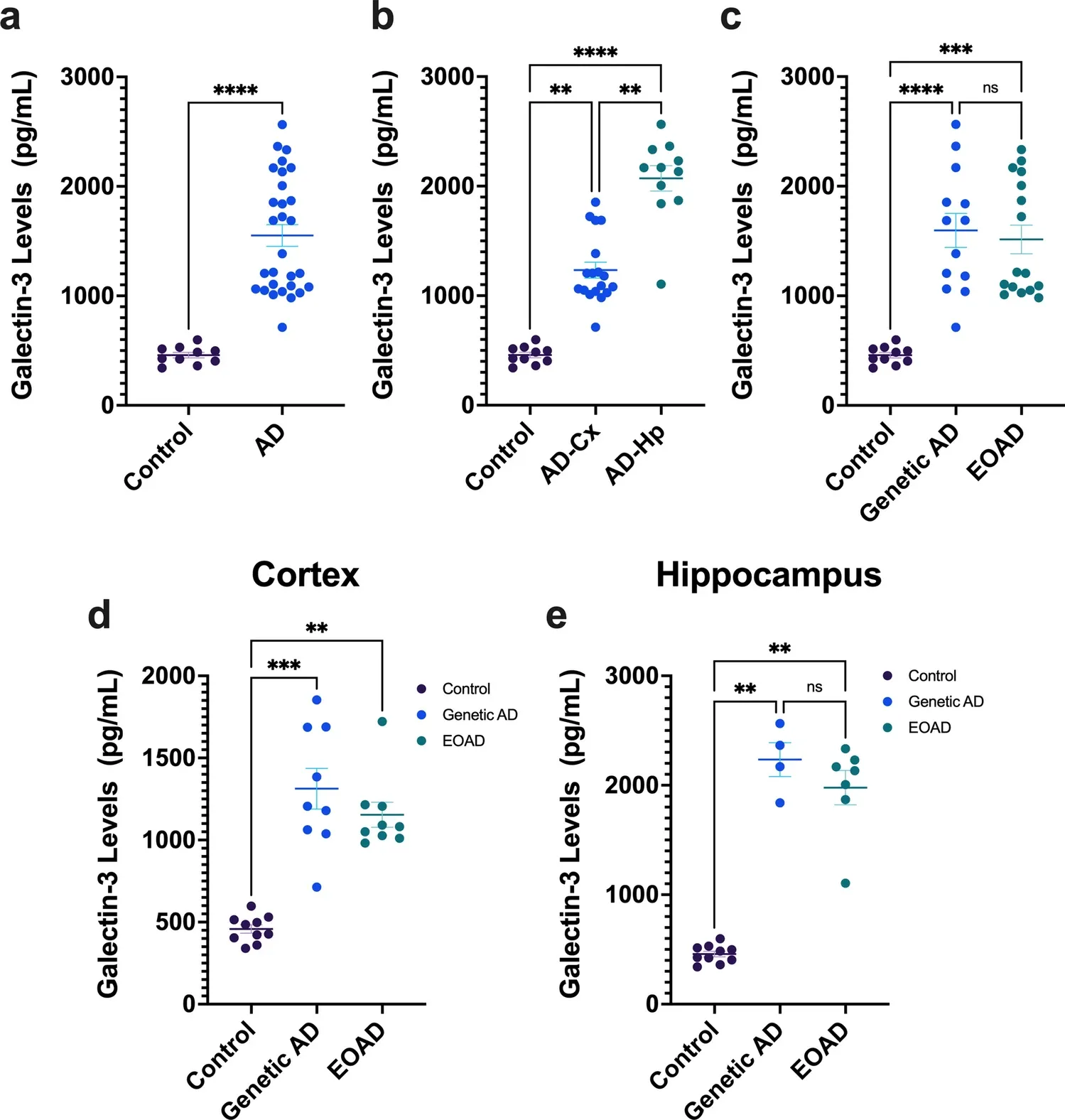
Galectin-3 is elevated in CSF and is associated with Abeta deposits and tau aggregates in brain tissue in Alzheimer's disease
https://link.springer.com/article/10.1007/s00401-022-02469-6
Tokenomics
10,000,000
Total SupplyIgnition Sale | 10% |
Wide Liquidity | 20% |
Concentrated Liquidity | 15% |
Cerebrum DAO | 35% |
Treasury | 12.5% |
Bio Protocol Call Option | 2.5% |
veBIO Airdrop | 5% |
10,000,000
Total SupplyIgnition Sale | 10% |
Wide Liquidity | 20% |
Concentrated Liquidity | 15% |
Cerebrum DAO | 35% |
Treasury | 12.5% |
Bio Protocol Call Option | 2.5% |
veBIO Airdrop | 5% |
Community
Stay up to date
Get the latest community updates and find out about upcoming Neuron-Gale developments